Understanding The Vraylar ICD-10 Code For Effective Coding And Billing
Decoding the Vraylar ICD-10 Code
Have you ever wondered what goes on behind the scenes when you visit your healthcare provider and they submit a claim to your insurance company for reimbursement? One crucial element in this process is the use of medical codes, specifically the ICD-10 codes. These codes are used to classify diseases, symptoms, abnormal findings, complaints, social circumstances, and external causes of injury or disease. In the realm of mental health, the Vraylar ICD-10 code plays a significant role in ensuring accurate billing and coding practices.
Vraylar, also known by its generic name cariprazine, is a medication commonly prescribed for the treatment of schizophrenia and bipolar disorder. It belongs to a class of medications called atypical antipsychotics, which are known for their efficacy in managing symptoms of these mental health conditions. When it comes to billing and coding for Vraylar, healthcare providers must use the appropriate ICD-10 code to indicate the reason for prescribing this medication.
The Vraylar ICD-10 code is crucial for accurately documenting the patient’s condition and ensuring that the insurance company processes the claim correctly. By using the correct code, healthcare providers can streamline the billing process and minimize the risk of claim denials or delays in reimbursement. This ultimately benefits both the healthcare provider and the patient, as it ensures that the patient receives the necessary treatment without any financial barriers.

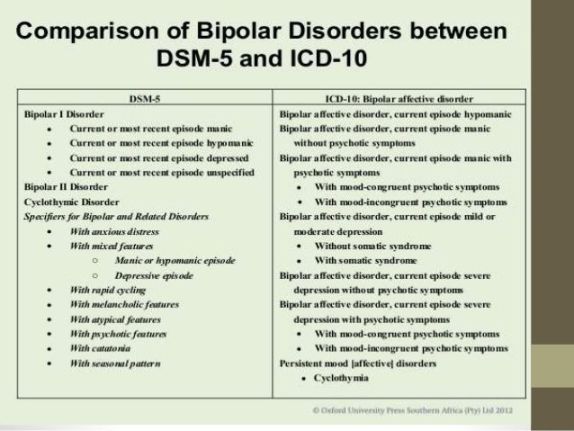
One of the most common ICD-10 codes used in conjunction with Vraylar is F31.81, which corresponds to bipolar disorder, current episode manic with mild severity. This code indicates that the patient is experiencing a manic episode of bipolar disorder and requires treatment with Vraylar to manage their symptoms. By using this code, healthcare providers can communicate to the insurance company the specific reason for prescribing Vraylar and justify the need for this medication in the patient’s treatment plan.
In addition to the F31.81 code, there are several other ICD-10 codes that may be used in conjunction with Vraylar, depending on the patient’s diagnosis and symptoms. For example, the ICD-10 code F20.9 corresponds to schizophrenia, unspecified, while the code F31.9 indicates bipolar disorder, unspecified. Healthcare providers must carefully assess the patient’s condition and select the appropriate ICD-10 code that best describes the reason for prescribing Vraylar.

Understanding the Vraylar ICD-10 code is essential for healthcare providers to effectively code and bill for this medication. By accurately documenting the patient’s condition and using the correct code, healthcare providers can ensure that the insurance company processes the claim promptly and reimburses them for the services provided. This not only benefits the healthcare provider by ensuring proper reimbursement but also benefits the patient by facilitating access to the necessary treatment.
In conclusion, decoding the Vraylar ICD-10 code is a crucial step in the billing and coding process for healthcare providers prescribing this medication. By using the appropriate code to accurately document the patient’s condition, healthcare providers can streamline the billing process and ensure that the patient receives the necessary treatment without any financial barriers. Understanding the importance of the Vraylar ICD-10 code is key to effective coding and billing practices in the realm of mental health.